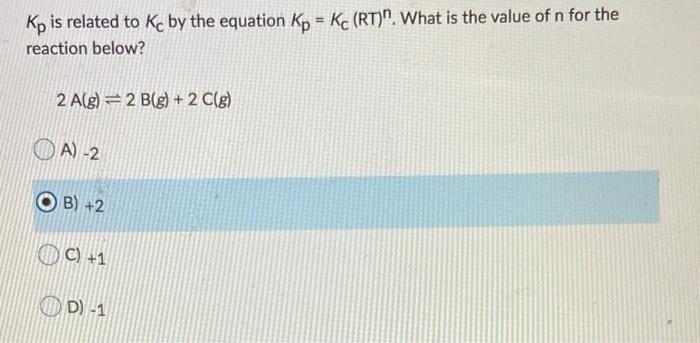
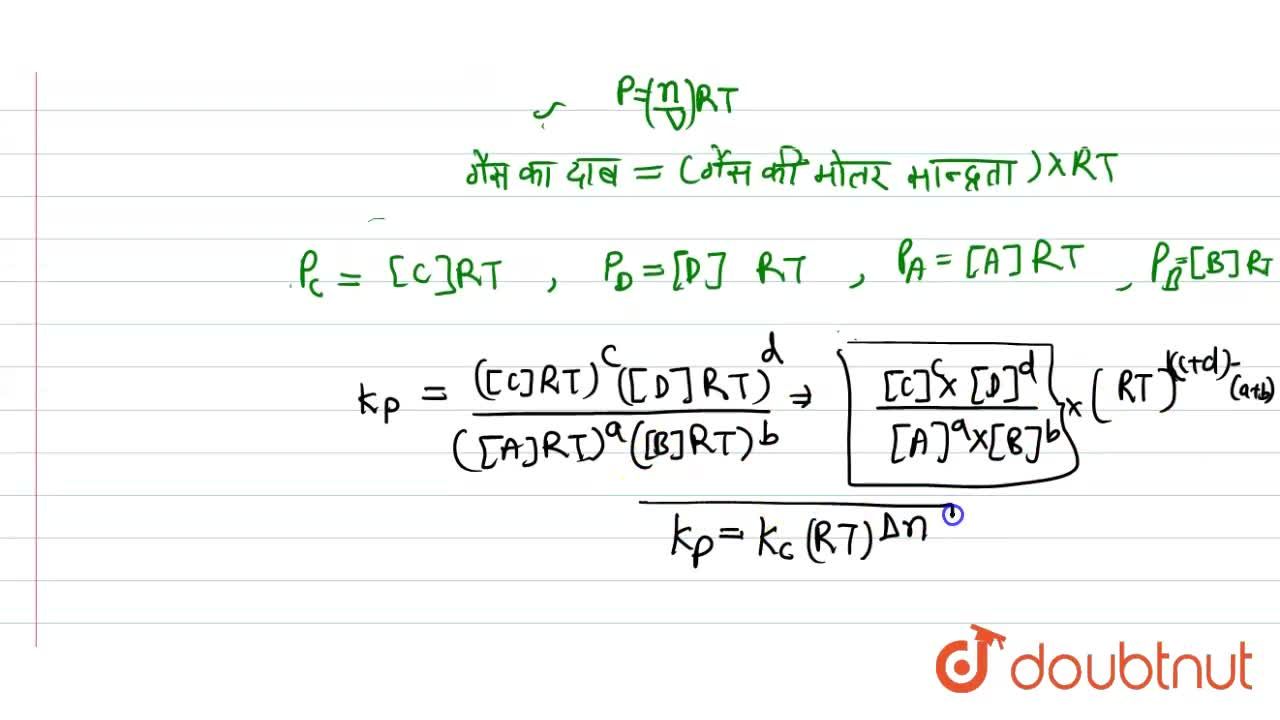
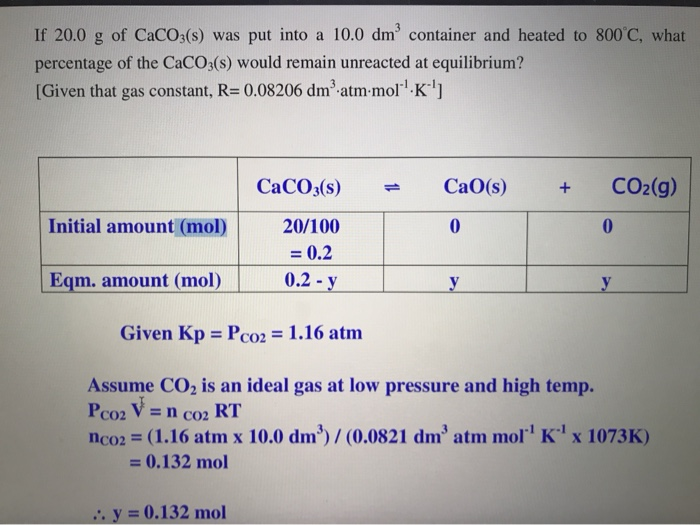
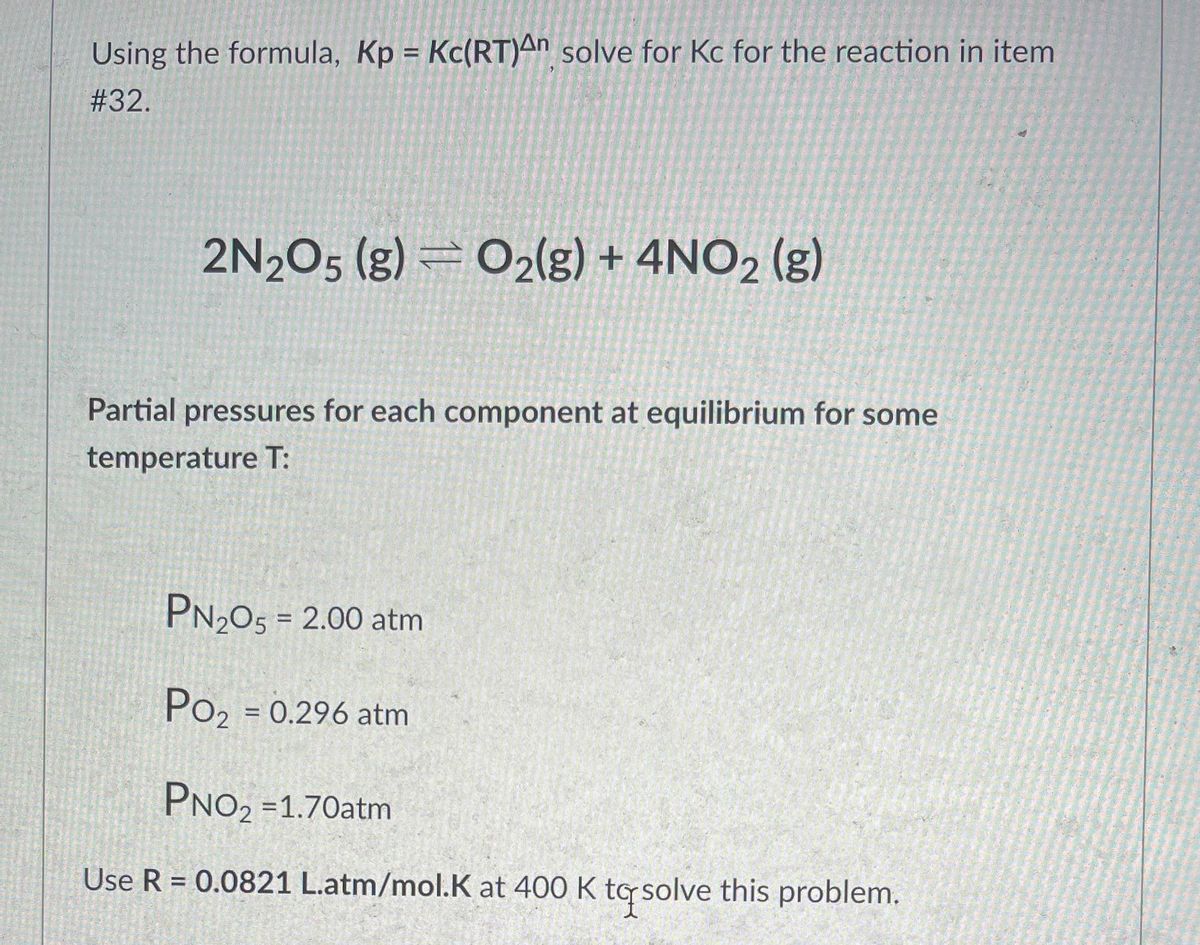
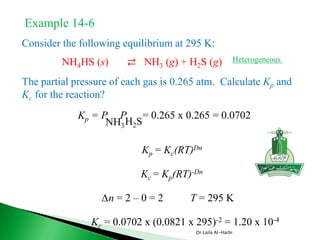
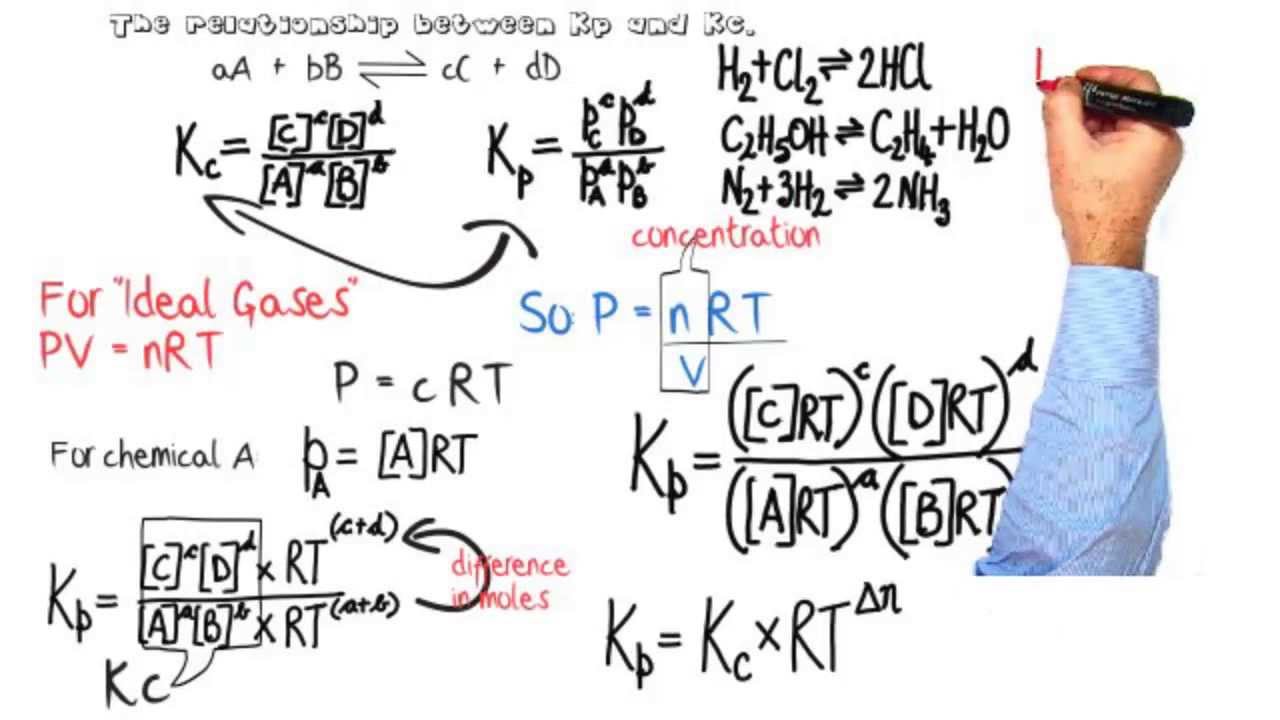
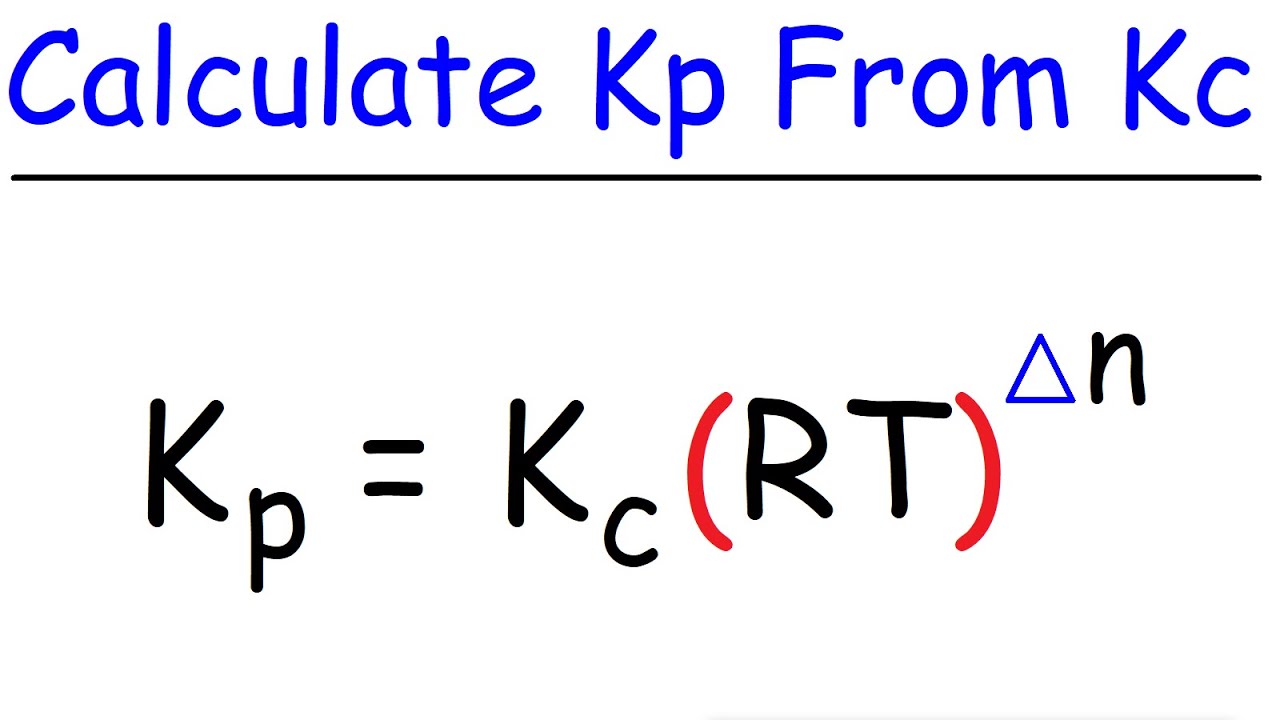
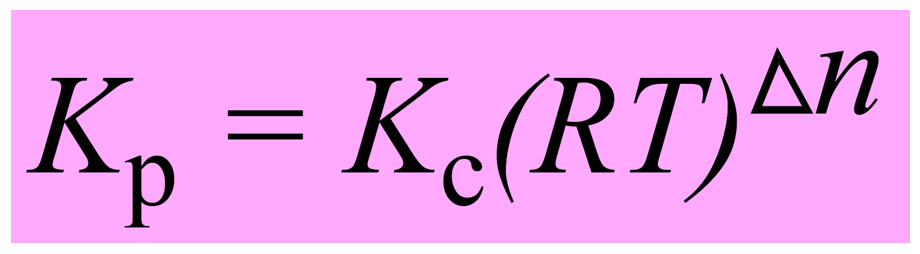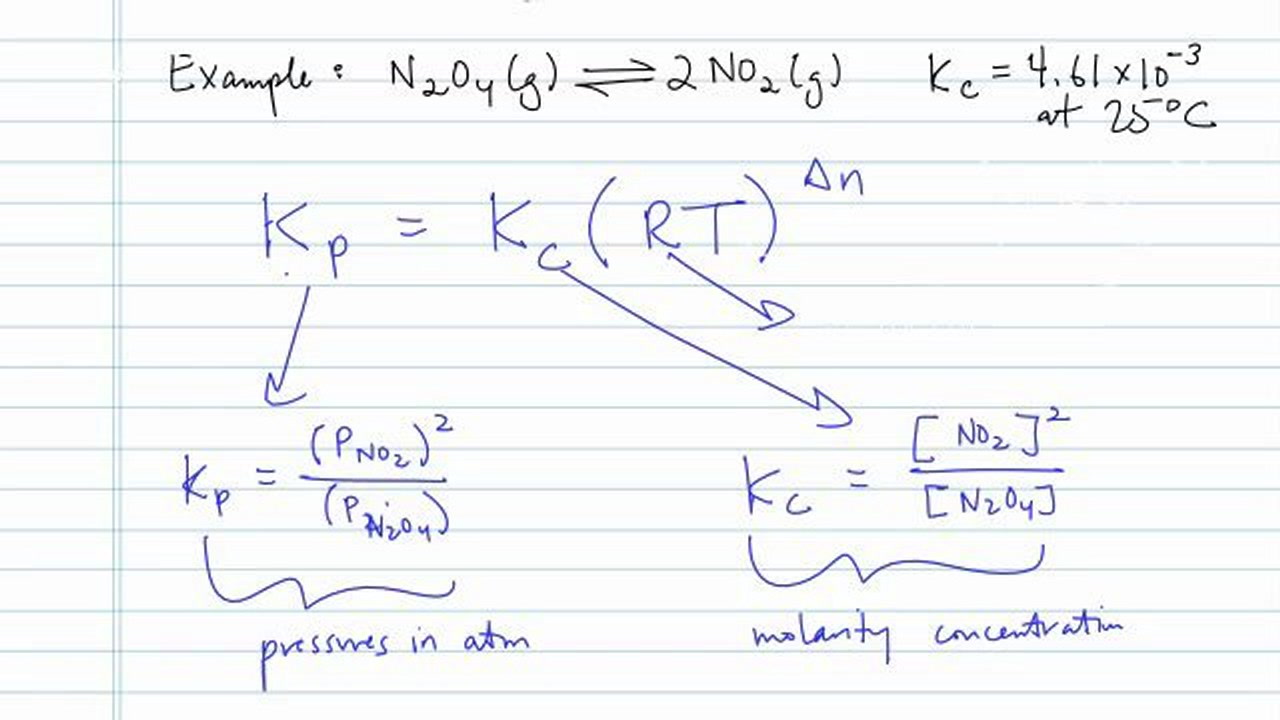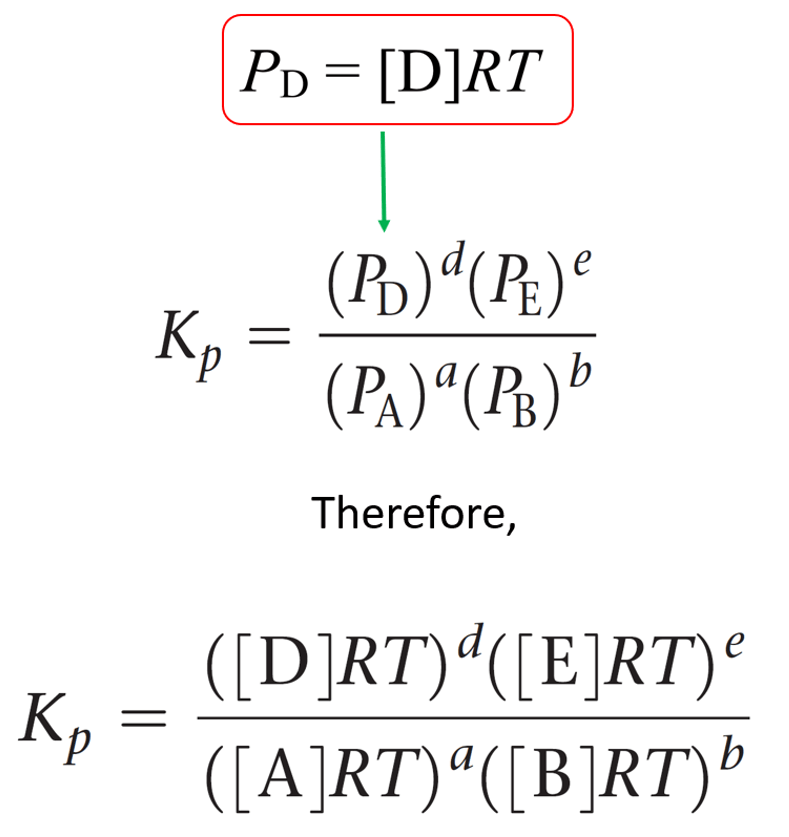SOLVED: The equilibrium constant, KcKcKc, is calculated using molar concentrations. For gaseous reactions another form of the equilibrium constant, KpKpKp, is calculated from partial pressures instead of concentrations. These two equilibrium constants

Plz explain in detail And also in the solution it states Kp=Kc, but that isn't possible rt cause it - Chemistry - The Solid State - 14388739 | Meritnation.com