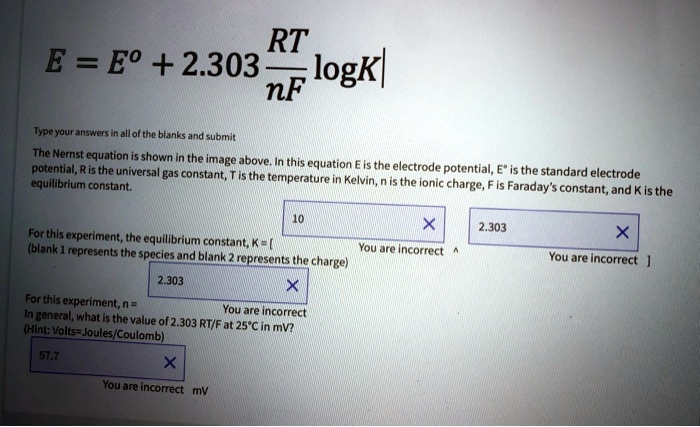
SOLVED: RT E = Eo + 2.303 logkl nF Typeyour. Kanshen allof the blanks and submit The Nernst equation shown the image above: this potential, R is the universa equation constant; is
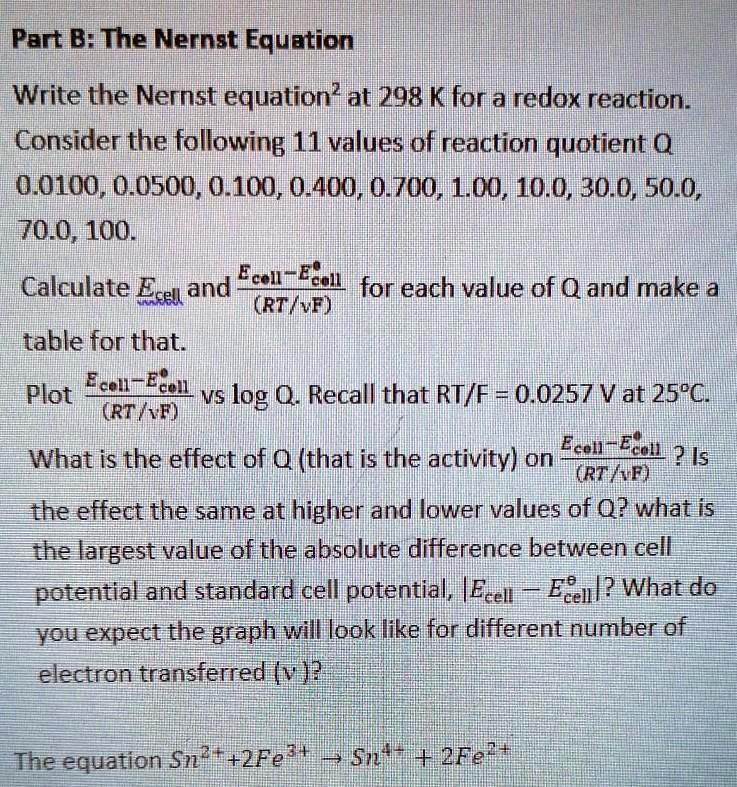
SOLVED: Part B: The Nernst Equation Write the Nernst equation?at 298 K for a redox reaction . Consider the following 11 values of reaction quotient 0 0.0100, 0.0500, 0.100, 0.400, 0.700, 1.00,,10.0,30.0,50.0,

What is the value of log Ksp for AgI, if: Ag + I^ - → AgI + e; E^o = 0.152 V Ag → Ag^ + + e ; E^o = - 0.800 V

Regression Analysis (Evaluate Predicted Linear Equation, R-Squared, F-Test, T-Test, P-Values, Etc.) - YouTube
FIGURE. Scatter plot of RTF values for cartoon (upper panel) and motion... | Download Scientific Diagram
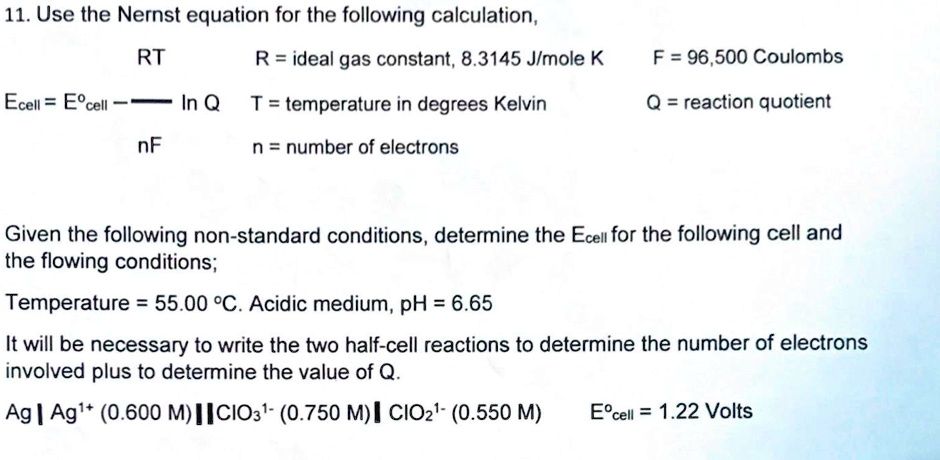
SOLVED: 11. Use the Nernst equation for the following calculation, RT R = ideal gas constant; 8.3145 Jlmole K F = 96,500 Coulombs Ecell = E%cell In Q T= temperature in degrees

![The emf of cell at 298 K is: [Given : 2.303 × RTF = 0.06] The emf of cell at 298 K is: [Given : 2.303 × RTF = 0.06]](https://dwes9vv9u0550.cloudfront.net/images/5237783/c7440e35-87aa-4c0d-aa35-770d84a298ba.jpg)

![The emf of cell at 298 K is: [Given : 2.303 × RTF = 0.06] The emf of cell at 298 K is: [Given : 2.303 × RTF = 0.06]](https://dwes9vv9u0550.cloudfront.net/images/2198016/49d1c614-8901-47ef-8a79-1474eae4931a.jpg)






![The emf of cell at 298 K is: [Given : 2.303 × RTF = 0.06] The emf of cell at 298 K is: [Given : 2.303 × RTF = 0.06]](https://dwes9vv9u0550.cloudfront.net/images/392604/440d00d5-9b20-4a51-a7cd-142252fa7105.jpg)
